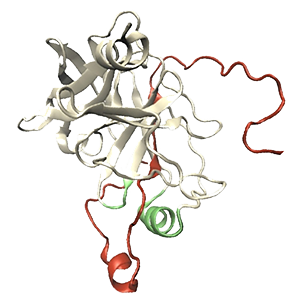
Using molecular dynamics simulations, we describe how crowded environments affect the internal dynamics and diffusion of the hepatitis C virus proteases NS3/4A. This protease plays a key role in viral replication and is successfully used as a target for antiviral treatment. The NS3 enzyme requires a peptide cofactor, called NS4A, with its central part interacting with the NS3 β-sheet, and flexible, protruding terminal tails that are unstructured in water solution. The simulations describe the enzyme and water molecules at atomistic resolution, whereas crowders are modeled via either all-atom or coarse-grained models to emphasize different aspects of crowding. Crowders reflect the polyethylene glycol (PEG) molecules used in the experiments to mimic the crowded surrounding. A bead-shell model of folded coarse-grained PEG molecules considers mainly the excluded volume effect, whereas all-atom PEG models afford more protein-like crowder interactions. Circular dichroism spectroscopy experiments of the NS4A N-terminal tail show that a helical structure is formed in the presence of PEG crowders. The simulations suggest that crowding may assist in the formation of an NS4A helical fragment, positioned exactly where a transmembrane helix would fold upon the NS4A contact with the membrane. In addition, partially interactive PEGs help the NS4A N-tail to detach from the protease surface, thus enabling the process of helix insertion and potentially helping the virus establish a replication machinery needed to produce new viruses. Results point to an active role of crowding in assisting structural changes in disordered protein fragments that are necessary for their biological function.
